Standard Reduction Potential : Solved: Given These Standard Reduction Potentials At 25 °C ... / When solving for the standard cell potential, the species oxidized and the species reduced must be identified.
Standard Reduction Potential : Solved: Given These Standard Reduction Potentials At 25 °C ... / When solving for the standard cell potential, the species oxidized and the species reduced must be identified.. At the top left of the table (where the green arrow is pointing) are the substances that are easiest to reduce. The more positive the potential is the more likely it will be … Identify the oxidizing and reducing. Reduction potential of metal reacting with cu (assumes that cu. Element, reaction equation and standard you are here:
Identifying trends in oxidizing and reducing agent strength. It is expressed in volts at standard conditions like. The standard reduction potential for a free co+3 ion is 1.853 v, while in complexed state co(nh3)6+3, it decreases to 0.1 v. Similarly, a free fe+3 ion has a standard reduction potential of. The standard reduction potential is defined relative to a standard hydrogen electrode (she) reference electrode, which is arbitrarily given a potential of 0.00 volts.

Identifying trends in oxidizing and reducing agent strength.
A standard electrode potential1 is a measure of the energy per electron in a given reaction. When solving for the standard cell potential, the species oxidized and the species reduced must be identified. To calculate the standard cell potential for a reaction. How to use a table of standard reduction potentials to calculate standard cell potential. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Identify the oxidizing and reducing. Redox potential (also known as oxidation / reduction potential, 'orp', pe, e0', or. The standard reduction potential is defined relative to a standard hydrogen electrode (she) reference electrode, which is arbitrarily given a potential of 0.00 volts. The standard reduction potential for the r /r couple is about 1.26 v at 25°c. This video is about electrochemistry and explains in details the standard reduction potential. The table of standard redox potentials for total and inorganic chemistry contains: Standard electrode potentials for reduction and oxidation reactions tutorial.
A list of common standard reaction potentials is given below Students studying chemistry at different levels could highly. The standard reduction potential is defined relative to a standard hydrogen electrode (she) reference electrode, which is arbitrarily given a potential of 0.00 volts. The more positive the potential is the more likely it will be … Standard reduction potentials at 25oc*.

Standard reduction potential is characterized comparative to a standard hydrogen electrode (she) as a reference electrode having a potential 0.00v.
) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and. The order of the metals in results table 2 the same as that in the table of standard reduction potentials on p 833 in zumdahl's text? Choose from 322 different sets of flashcards about standard reduction potentials on quizlet. When solving for the standard cell potential, the species oxidized and the species reduced must be identified. The standard reduction potential for the r /r couple is about 1.26 v at 25°c. The standard reduction potential is defined relative to a standard hydrogen electrode (she) reference electrode, which is arbitrarily given a potential of 0.00 volts. How to use a table of standard reduction potentials to calculate standard cell potential. Standard reduction potentials are used to determine the standard cell potential. A list of common standard reaction potentials is given below A standard electrode potential1 is a measure of the energy per electron in a given reaction. The standard reduction potential for a free co+3 ion is 1.853 v, while in complexed state co(nh3)6+3, it decreases to 0.1 v. The standard reduction potential is the likelihood of being reduced (accept electrons.) higher the value, easily reduced. Standard reduction potential is characterized comparative to a standard hydrogen electrode (she) as a reference electrode having a potential 0.00v.
A standard electrode potential1 is a measure of the energy per electron in a given reaction. We will consider a simple reversible redox reaction more interested in redox potential values that are more representative of typical natural. Reduction potential of metal reacting with cu (assumes that cu. All standard potentials are reduction potentials unless told otherwise. When solving for the standard cell potential, the species oxidized and the species reduced must be identified.
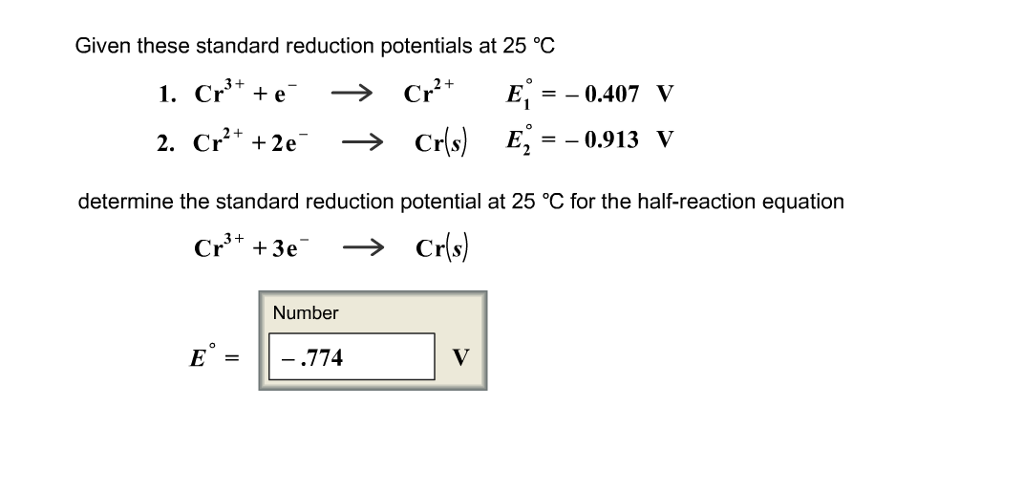
Element, reaction equation and standard you are here:
A list of common standard reaction potentials is given below The more positive the potential is the more likely it will be … Learn about standard reduction potentials with free interactive flashcards. A standard electrode potential1 is a measure of the energy per electron in a given reaction. This video is about electrochemistry and explains in details the standard reduction potential. Standard electrode potentials for reduction and oxidation reactions tutorial. Choose from 322 different sets of flashcards about standard reduction potentials on quizlet. The standard reduction potential is the likelihood of being reduced (accept electrons.) higher the value, easily reduced. Table of common standard reduction potentials. When solving for the standard cell potential, the species oxidized and the species reduced must be identified. The table of standard redox potentials for total and inorganic chemistry contains: Similarly, a free fe+3 ion has a standard reduction potential of. Standard reduction potentials at 25oc*.

Kommentare
Kommentar veröffentlichen